Global Neurological Biomarkers Market to Reach USD 9.91 Billion by 2030
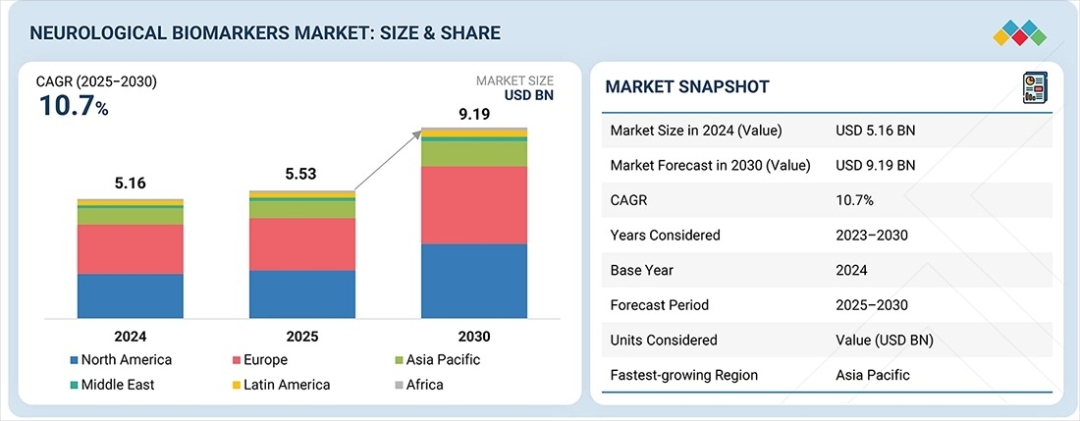
The global neurological biomarkers market is entering a high-growth phase that is reshaping the future of neurology, drug development, and precision medicine. Valued at USD 5.53 billion in 2025, the market is projected to reach USD 9.91 billion by 2030, expanding at a compelling CAGR of 10.7% during the forecast period.
What is fueling this acceleration, and why should executive leadership pay attention now? The answer lies in the convergence of rising neurological disease burden, biomarker-guided clinical decision-making, and rapid technological advancements in genomics, proteomics, and digital health.
Download PDF Brochure:https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=153432005
Market Overview
Neurological biomarkers are measurable biological characteristics used to diagnose, characterize, prognosticate, and guide therapeutic management of diseases affecting the central and peripheral nervous systems. As healthcare systems shift toward precision neurology, the clinical adoption of biomarker-guided diagnosis and treatment is expanding significantly.
How are these biomarkers transforming care? They enable earlier detection, more accurate disease monitoring, and targeted therapeutic strategies for neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease. Increasing use of DNA-, RNA-, and protein-based tests—alongside specific markers such as neurofilament light chain (NfL) and phospho-tau—is strengthening clinical confidence and accelerating demand across diagnostics and research environments.
Recent advances in next-generation sequencing, Simoa assays, and multiplex testing platforms are further enhancing analytical sensitivity and reproducibility. These innovations, adopted by pharmaceutical companies and specialized organizations, are improving drug development pipelines and delivering validated, reliable diagnostic outcomes.
Key Growth Drivers and Strategic Forces
The rising prevalence and diagnosis rates of neurological disorders—including Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, epilepsy, stroke, and traumatic brain injury—are central to market expansion. As patient populations grow globally, healthcare providers increasingly require reliable biomarkers to support early diagnosis, disease stratification, and longitudinal monitoring.
At the same time, pharmaceutical and biotechnology firms are integrating neurological biomarkers into clinical trials to enhance patient selection, optimize endpoints, and reduce development risk. Who benefits from this integration? Drug developers, contract research organizations, diagnostic laboratories, and ultimately patients seeking more precise treatment pathways.
However, the market also faces structural constraints. High development costs associated with advanced assays, imaging procedures, and analytical platforms can limit adoption, particularly in resource-constrained healthcare systems. For CFOs and procurement leaders, cost-effectiveness and reimbursement alignment remain critical considerations.
Emerging Opportunities in Digital and Computational Biomarkers
The expansion of digital health technologies is opening a new frontier in neurological biomarkers. Wearable sensors, remote monitoring systems, and AI-driven analytics are enabling the creation of digital and computational biomarkers that capture continuous, real-world neurological function.
How does this shift impact stakeholders? Digital biomarkers offer longitudinal, scalable, and patient-centric data streams, supporting earlier intervention, decentralized trials, and outcome-based care models. As digital infrastructure matures, integration between biological and computational biomarkers is expected to redefine neurological assessment and monitoring frameworks.
Regulatory and Validation Complexities
Despite strong innovation momentum, regulatory and validation requirements remain a significant challenge. Demonstrating analytical validity, clinical relevance, reproducibility, and longitudinal reliability across heterogeneous patient populations requires rigorous testing and extended timelines.
Why does this matter for strategic planning? Regulatory barriers increase development costs and delay commercialization, influencing time-to-market strategies and capital allocation decisions. Navigating FDA and EMA validation standards is essential for companies seeking competitive positioning in Alzheimer’s and Parkinson’s diagnostics.
Collaborative Ecosystem Driving Innovation
The neurological biomarkers market functions within a collaborative ecosystem comprising diagnostic developers, laboratories, pharmaceutical companies, CROs, service providers, and patients. Companies are developing ultra-sensitive tools capable of detecting proteins such as neurofilament light chain in blood, while others have secured regulatory approvals for diagnostic tests aligned with stringent global standards.
Laboratories and hospitals are applying these solutions to optimize therapeutic decisions, while pharmaceutical firms embed biomarker endpoints into clinical development programs to improve drug discovery outcomes and trial efficiency.
This ecosystem also intersects with broader innovation domains, including drug discovery services, bioinformatics, genomics, AI in healthcare, and healthcare IT integration. Industry leaders can explore deeper insights into adjacent innovation landscapes through the following strategic resources:
By offering, consumables account for the largest share of the neurological biomarkers market. Their essential role in high-volume testing workflows for neuronal damage detection, neurodegeneration monitoring, and neuroinflammation assessment ensures recurring demand across clinical and research settings.
By type, proteomic biomarkers dominate due to their strong clinical relevance in identifying neuronal injury and inflammatory processes. Their diagnostic utility in neurodegenerative conditions continues to drive widespread adoption.
By application, Alzheimer’s disease represented the largest segment in 2024. This leadership position reflects the growing global disease burden, heightened demand for early and accurate diagnosis, adoption of fluid-based biomarkers targeting amyloid and tau pathology, and increasing integration into clinical trials.
From an end-user perspective, pharmaceutical and biotechnology companies represent the largest segment. Their extensive reliance on neurological biomarkers for target validation, patient stratification, drug efficacy monitoring, and trial optimization underscores the market’s strategic importance to R&D pipelines.
Regional Outlook
North America currently holds the largest overall market share, supported by advanced healthcare infrastructure and strong research investment. However, Asia Pacific is projected to be the fastest-growing region during the forecast period.
What is driving growth in Asia Pacific? A rapidly aging population, rising prevalence of neurological disorders, expanding diagnostic infrastructure, and increasing government healthcare investments are key catalysts. Growing pharmaceutical and biotechnology R&D activity and the adoption of advanced biomarker technologies across China, Japan, India, and Southeast Asia are further accelerating regional momentum.
For multinational companies, Asia Pacific represents both a growth opportunity and a strategic expansion corridor.
Request Sample Pages-https://www.marketsandmarkets.com/requestsampleNew.asp?id=153432005
Competitive Landscape
The neurological biomarkers market features prominent industry leaders including ABBOTT (US), THERMO FISHER SCIENTIFIC INC. (US), QIAGEN (Germany), BIO-RAD LABORATORIES, INC (US), MERCK KGaA (Germany), JOHNSON & JOHNSON (US), and QUANTERIX (US). These companies are investing in assay innovation, advanced analytical platforms, regulatory approvals, and global expansion strategies to strengthen market position.
Why This Matters Now
As neurological disorders rise globally and precision medicine becomes a strategic healthcare priority, neurological biomarkers are transitioning from research tools to core clinical and commercial assets. For CEOs, CFOs, and strategic leaders, investment decisions in biomarker technologies directly influence competitive differentiation, pipeline acceleration, and long-term value creation.
Organizations that align early with validated, scalable biomarker solutions will be better positioned to navigate regulatory complexity, capture emerging digital opportunities, and meet growing demand for precision neurology through 2030 and beyond.
Media Contact
Company Name: MarketsandMarkets™ Research Private Ltd.
Contact Person: Mr. Rohan Salgarkar
Email: Send Email
Phone: 18886006441
Address:1615 South Congress Ave. Suite 103, Delray Beach, FL 33445
City: Florida
State: Florida
Country: United States
Website: https://www.marketsandmarkets.com/
Press Release Distributed by ABNewswire.com
To view the original version on ABNewswire visit: Global Neurological Biomarkers Market to Reach USD 9.91 Billion by 2030
Information contained on this page is provided by an independent third-party content provider. XPRMedia and this Site make no warranties or representations in connection therewith. If you are affiliated with this page and would like it removed please contact [email protected]